New anti-viral based therapies for Covid-19: Protease inhibitors
Researchers at MWC are looking to develop novel inhibitors for SARS-CoV-2 Mpro by leveraging expertise within MWC’s Infectious Disease network for anti-viral drug development.
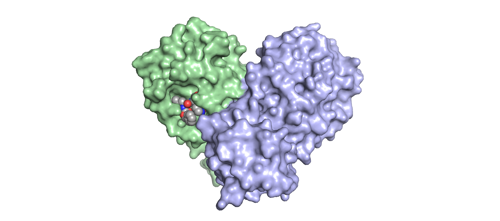
A model of the Mpro protein. The protein is coloured green (subunit A) and blue (subunit B) and the control inhibitor NV-001 (aka GC373) is in the active site of subunit A (shown as spheres, coloured by atom red=oxygen, blue=nitrogen, Grey=carbon). Image courtesy of Dr Ashley Campbell.
Strong collaboration among specialist research groups, MWC Principal Investigators and Early Career Researchers has enabled an iterative process generating a pipeline of promising novel compounds in the ongoing fight against SARS-CoV-2 — and wider preparedness against future viral pandemics.
Dr Yann Hermant, Research Fellow in the laboratory of Distinguished Professor Dame Margaret Brimble at the University of Auckland, Dr Ashley Campbell, Postdoctoral Fellow in the laboratory of Professor Kurt Krause and Dr Alice McSweeney, Postdoctoral Fellow in Professor Vernon Ward’s group, at the University of Otago, are leading the project investigating potential inhibitors for the Mpro protein from the SARS-CoV-2 virus. MWC has supported a portion of the salaries for Yann and Ashley.
The protein ‘Mpro’ is essential in the replication cycle of the SARS-CoV-2 virus and is an important biological target for the development of drugs to treat SARS-CoV-2 and other viruses. The most advanced SARS-CoV-2 Mpro inhibitor (Paxlovid/PF-07321332) gained emergency use authorisation for treating COVID-19 patients with a high risk of disease progression. The MWC-enabled cross-institutional team aims to find complementary Mpro inhibitors to accelerate second-generation therapies and prepare against potential resistance.
Ashley, a structural biologist, says their work is part of an exciting global race to find and patent anti-viral therapies that are both effective and unique.
Yann, a medicinal chemist, synthesises novel compounds designed to block the Mpro protein’s activity. These new inhibitors are sent to the University of Otago, where Alice conducts functional assays to test each new compound’s ability to inhibit Mpro function. The most active contenders are then provided to Ashley, who uses x-ray crystallography to generate 3D models of each compound’s structure bound to the Mpro target protein at atomic resolution. These 3D models illuminate in detail the interactions between the drug and the protein.
Yann says the detailed models help increase understanding about what drives tight binding interactions, “whether that’s an accessible atom, a charged surface, or the constraint of space where a large molecule caused the enzyme to change its shape to accommodate its bulk”. Together with information from the functional assays, the atomic models give insights into how to redesign compounds to generate even tighter binding.
The researchers send the most promising compounds to the US National Institute of Allergy and Infectious Diseases, where they are tested on variants of SARS-CoV-2 and other viruses, such as norovirus. “That is really exciting because then we may discover that we have a broad-spectrum anti-viral,” says Ashley.
The Mpro project is encompassed within a much larger MWC-facilitated endeavour. The goal is to build a platform to generate these anti-viral compounds with a team of New Zealand scientists who can respond swiftly in the face of any viral pandemic or epidemic outbreak.
Yann says, “It is important to have this network established and working as there are a lot of viruses slowly creeping toward New Zealand. For instance, with global warming allowing insects like mosquitoes to spread, we may start seeing viruses like the Dengue virus that were previously not seen in our country.”