Following the evolving genetics of cancer
Through a broad transdisciplinary project and extensive DNA sequencing analysis, MWC scientists have identified how different metastases evolve through a variety of genetic changes, including how they adapt to avoid the immune system.
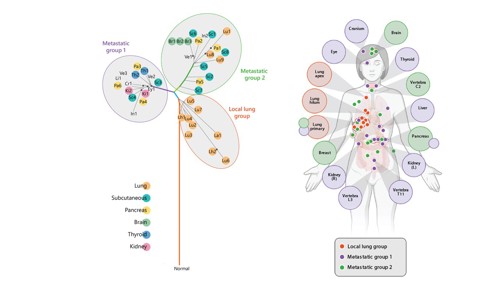
Left: DNA phylogram displaying the major anatomical locations. Coloured circles behind sample names indicate major anatomical locations according to the key (bottom left). Right: Anatomic location of genomic groups. Colored circles overlaid on body represent tumor sampling sites colored according to the genomic group(s) present. Tumors are labeled and shaded according to dominant genomic group(s). Images from Robb (2021) and Robb et al (2023), https://doi.org/10.1158/2767-9764.CRC-22-0101.
The Rapid Autopsy study, led by MWC Associate Investigators oncologist Ben Lawrence and scientist Cherie Blenkiron, along with MWC Principal Investigator Cris Print, has given insight into how scientists and clinicians can follow evolving cancers by sequencing mutations that leak out of tumours into the blood, and how tumour heterogeneity can influence treatment strategies.
Tumour heterogeneity is the emerging idea that not all cells in a tumour, or even tumours around a body, have the same genetic profile.
MWC supported this research from its early beginnings by supporting development of the tissue science and molecular technologies required for this work and access to molecular databases required for data interpretation, as well as supporting MWC investigators and researchers across the project.
Professor Print says the study has been a true team journey with the tissue donor, her whānau , PhD candidates, oncologists, radiologists, pathologists, laboratory and bioinformatic scientists and even virtual reality visualisation experts from the University of Auckland’s Centre for eResearch and School of Architecture.
The donor, a woman nearing the end of her battle with cancer, had the foresight and tenacity to recognise the value her tumours might offer others after her death. Her gift has provided an unparalleled opportunity to investigate many tumours at high resolution, something not possible during a patient’s lifetime. Her whānau remain engaged with the research group and keep up to date with the scientific discoveries which their mother’s gift continues to deliver in aiding the understanding of cancer.
Dr Tamsin Robb, now a postdoctoral fellow in the Print and Lawrence Group’s has been a lynchpin across the project. While exploring the metastases from the patient within her PhD research, she uncovered a Darwinian-like evolution of cancer cells. By tracking a stepwise series of mutations for 44 tumours, Dr Robb discovered that it was not the mutations that drove the tumour growth initially.
“We saw base-pair mistakes in the ARID1A gene in every sample analysed at autopsy. We would expect this to be an early event in the tumours’ evolutionary history – but paradoxically, the ARID1A variant was not present in the tumour when the patient was first diagnosed.”
Instead, Dr Robb saw the loss and gain of copies of different chromosomes without any recognised mutation were driving the tumour initially – the mutations came later. Tamsin also saw that blood samples showed some mutations, but not all. The patient had tumours in many sites including the lung, pancreas, brain, and subcutaneous tissue, yet not all these anatomical sites were equally detected in the blood plasma. Some metastases hide!
Professor Print says this is a new and exciting understanding of how tumours grow.
“This has big implications in how we treat cancers and may be why cancers metastasize in the first place or why tumours evade a drug treatment.”
The work was published in early 2023 in Cancer Research Communications1 and amassed a generous repository of data available for future research.