Drug selection a milestone for spin-out (2010)
In 2010 Maurice Wilkins Centre investigators Professors Bill Denny and Peter Shepherd selected a new anticancer drug candidate for clinical development, triggering US $4.5 million investment in the pharmaceutical company they founded two years earlier.
Professors Bill Denny and Peter Shepherd established Pathway Therapeutics in New Zealand to discover and develop inhibitors of phosphatidylinositol-3-kinase (PI3K) to treat cancer and inflammatory disease.
The scientists credit the Maurice Wilkins Centre with bringing their research groups together. Bill is a medicinal chemist who co-directs the Auckland Cancer Society Research Centre while Peter is an expert in cell signalling.
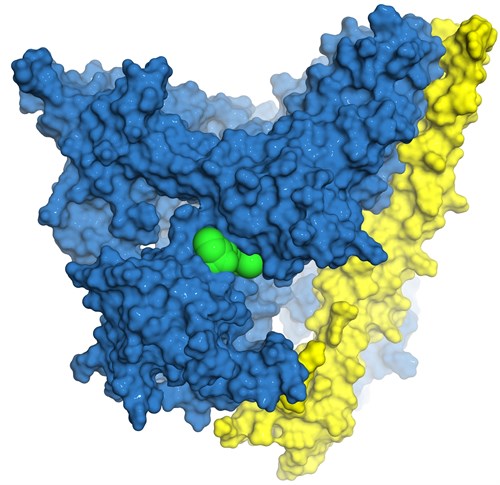 They collaborate on creating new drugs to inhibit PI3K, a family of four signalling proteins (alpha, beta, gamma and delta) that controls critical cell processes, from growth and proliferation to metabolism and survival.
They collaborate on creating new drugs to inhibit PI3K, a family of four signalling proteins (alpha, beta, gamma and delta) that controls critical cell processes, from growth and proliferation to metabolism and survival.
Abnormal PI3K activity is implicated in several diseases, including cancer, and PI3K inhibition is a “hot topic” in international cancer research.
Mutations in PI3K alpha are some of the most common genetic abnormalities in cancer, with errors or multiple copies of the gene found in a wide range of tumour types such as breast, colon, and lung cancers.
The new drug candidate, PWT33597, is a dual inhibitor of PI3K alpha and mTOR, one of the most important molecules activated by PI3K. Inhibiting both proteins provides a backup should cancer cells find a way to overcome either one of the blockades.
Laboratory research completed by the New Zealand scientists in 2010 confirmed that PWT33597 is selective for its targets, and very effectively inhibits the signalling pathway they regulate. It also showed that the compound is suitable for oral administration, achieving excellent activity at doses that seem suitable for use in patients.
Pathway Therapeutics announced that the investment triggered by the selection of PWT33597 will be used to advance the compound to clinical trials. PWT33597 is currently in preclinical development and an Investigational New Drug application is anticipated in 2011.
While PWT33597 is their most advanced compound, Pathway Therapeutics has several other PI3K inhibitors in the pipeline and the funds will support further research on these candidates overseas.
“Our work also demonstrates how commercially-focused research can synergise with traditional scientific outcomes,” says Peter. “It has already led to two successful grant applications to the Health Research Council of New Zealand as well as eleven scientific papers and many more to come.”
The Maurice Wilkins Centre has provided long-term salary support for two key scientists in the PI3K inhibitor programme and purchased a computer-controlled robot critical for screening the drug candidates.
Image: Representation of the 3D structure of the P13K protein (blue) bound to the regulartory subunit p85 (yellow) with the compound A66 (green) modelled in the nucleotide binding site of the P13K alpha kinase domain. Image courtesy of Dr Jack Flanagan (Auckland Cancer Society Research Centre).