Cancer drug advances to phase II clinical trials
A New Zealand cancer drug developed by two Maurice Wilkins Centre investigators has reached the second phase of clinical trials with patients.
Associate Professor Adam Patterson and Dr Jeff Smaill from the Auckland Cancer Society Research Centre and Maurice Wilkins Centre have collaborated with leading American biotechnology company Threshold Pharmaceuticals to advance a new cancer drug to phase II clinical trials. 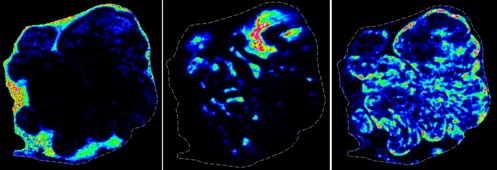
“The phase II clinical trials of TH-4000 are a culmination of over ten years of research and development,” says Adam. “It’s exciting to see a New Zealand developed cancer drug advance to this stage.”
The scientists have cleverly exploited an abnormality of tumours to make a drug that can discriminate between cancer and healthy tissue, potentially minimising the side effects of treatment. Administered in inactive ‘prodrug’ form, TH-4000 transforms into a cancer drug only in the abnormally low-oxygen (hypoxic) conditions found in most solid tumours. The treatment is known as a hypoxia-activated EGFR-TKI therapy.
Adam explains: “The drug targets the human epidermal growth factor receptor (EGFR) which is mutated or overactive in many cancers. Once active in the hypoxic zone, the drug inhibits the EGFR enzyme at its active site preventing it from sending signals that prompt a cancer cell to divide and grow.”
Current EGFR-TKI therapies have demonstrated significant clinical activity in non-small cell lung cancer that expresses mutant forms of EGFR. They have proven less effective in cancers expressing high levels of wild type EGFR, likely due to insufficient therapeutic index with dose-limiting toxicities causing side effects such as rashes or diarrhoea.
“TH-4000 is a molecularly-targeted, hypoxia-activated irreversible EFGR-TKI,” says co-inventor Jeff Smaill. “The drug is therefore expected to deliver greater efficacy with fewer side effects than available treatments.”
TH-4000 has shown positive results in limiting tumour growth in experimental models of non-small cell lung cancer (NSCLC) and head and neck cancers. Lung cancer is New Zealand’s leading cause of cancer deaths for men, and the second most common for women. NSCLC is the most common form and makes up approximately 80 per cent of lung cancers.
Both Adam and Jeff paid tribute to their talented drug development team. “It’s exciting for us to be working on this next generation approach of combining molecular and hypoxia targeting in a single drug candidate. We were particularly pleased to see the first two U.S. patents protecting TH-4000 issued by the U.S. Patent Office in 2015,” says Jeff.
Image: MALDI images of three small molecules that show distinct regional differences within a model solid tumour. In addition, the relative concentrations of each small molecule vary with position in the tumour microenvironment (dashed line indicates tissue edge).
Image courtesy of Angus Grey/Adam Patterson/Jeff Smaill.